In spring-summer 2020, Georgia Tech astrobiologists teamed up to create an in-house test kit to boost testing supplies. Read the NASA press release here.

Pre-print posted to MedRxiv on July 31, 2020:
SJ Mascuch, S Fakhretaha-Aval, JC Bowman, MTH Ma, G Thomas, B Bommarius, C Ito, L Zhao, GP Newnam, KR Matange, HR Thapa, B Barlow, RK Donegan, NA Nguyen, EG Saccuzzo, CT Obianyor, SC Karunakaran, P Pollet, B Rothschild-Mancinelli, S Mestre-Fos, R Guth-Metzler, AV Bryksin, AS Petrov, M Hazell, CB Ibberson, PI Penev, RG Mannino, WA Lam, AJ Garcia, JM Kubanek, V Agarwal, NV Hud, JB Glass, LD Williams, RL Lieberman. Buzz about RT-qPCR: An RT-qPCR formulation for SARS-CoV-2 detection using reagents produced at Georgia Institute of Technology. MedRXiv [link]
Widespread testing for the presence of novel coronavirus SARS-CoV-2 in patients remains vital for controlling the COVID-19 pandemic prior to the advent of an effective treatment. The early testing shortfall in some parts of the US can be traced to an initial shortage of supplies, expertise and/or instrumentation necessary to detect the virus by quantitative reverse transcription polymerase chain reaction (RT-qPCR). Here we show that academic biochemistry and molecular biology laboratories equipped with appropriate expertise and infrastructure can produce the RT-qPCR assay and backfill pipeline shortages. The Georgia Tech COVID-19 Test Kit Support Group synthesized multiplexed primers and probes and formulated a master mix composed of enzymes and proteins produced in-house. We compare the performance of our in-house kit to a commercial product used for diagnostic testing and describe implementation of environmental testing to monitor surfaces across various campus laboratories for the presence of SARS-CoV-2.
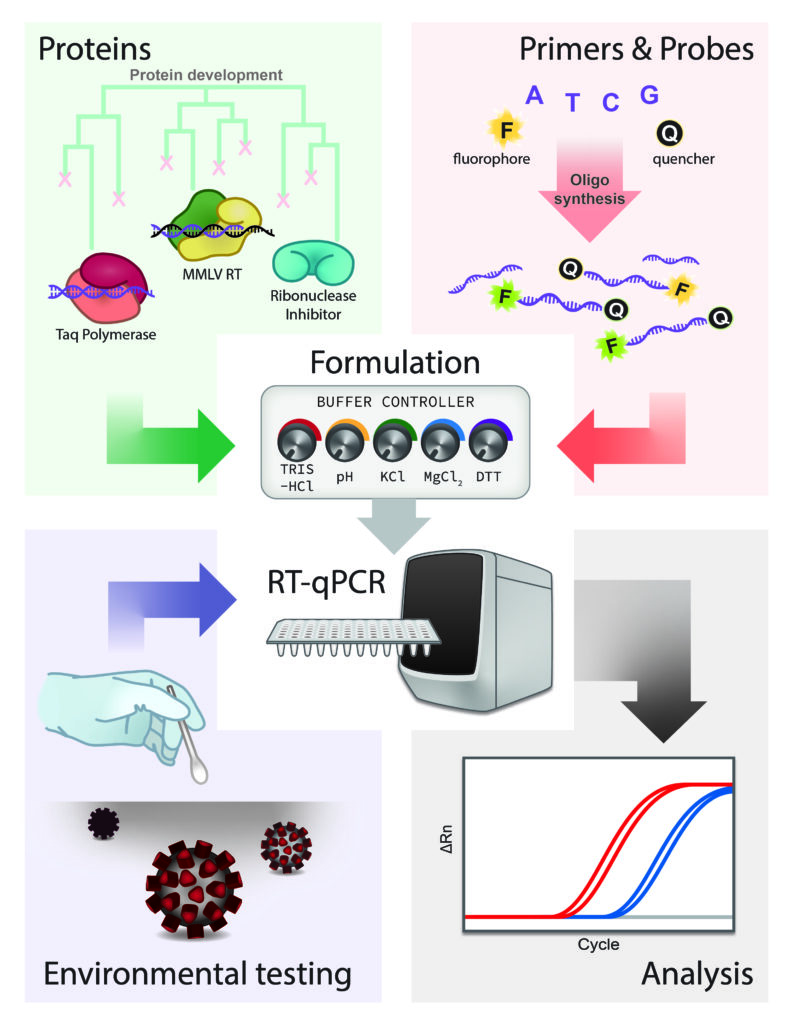
Image by Rebecca Guth-Metzler, PhD candidate, advisors: Loren Williams and Jennifer Glass (Figure 1 in Mascuch et al. 2020, MedRXiv [link].
